
When the soap bubbles start to float up, hold a burning candle to them and listen carefully for the sound they make when they pop.The first few bubbles of gas that are released from the end of the delivery tube will be air. Add approximately 40 ml of hydrochloric acid and immediately place the stopper on the test tube.Place approximately 1 g of the magnesium pieces in the test tube, but do not add the hydrochloric acid until everything else is ready to be assembled.Hypothesising, preparing, measuring, observing, interpretingĪ possible extension is to hold a cold piece of metal or glass above the place where you burst the bubbles so that the water vapour that forms during the reaction condenses on the metal or glass. Investigation: The reaction between magnesium and hydrochloric acid Remembering, balancing chemical equations

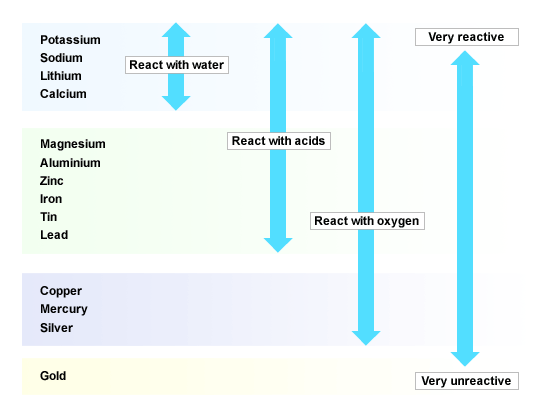
Seeing the real world application for what they learn in the classroom is a very important part of the learning process and in discovering what is possible through science and technology.ħ.1 The reaction of an acid with a metal (1.5 hours) Although this is not for assessment purposes, if you do not have time to do it in class, we encourage you to encourage or get your learners to do it as a homework activity. At the end of this chapter, there is a short activity on some of the careers in the chemical industry. The last reactions to look at are those between an acid and a metal.
#Reactivity of metals series
This is a short chapter to conclude the series of reactions that learners will have been exposed to this term.


 0 kommentar(er)
0 kommentar(er)
