
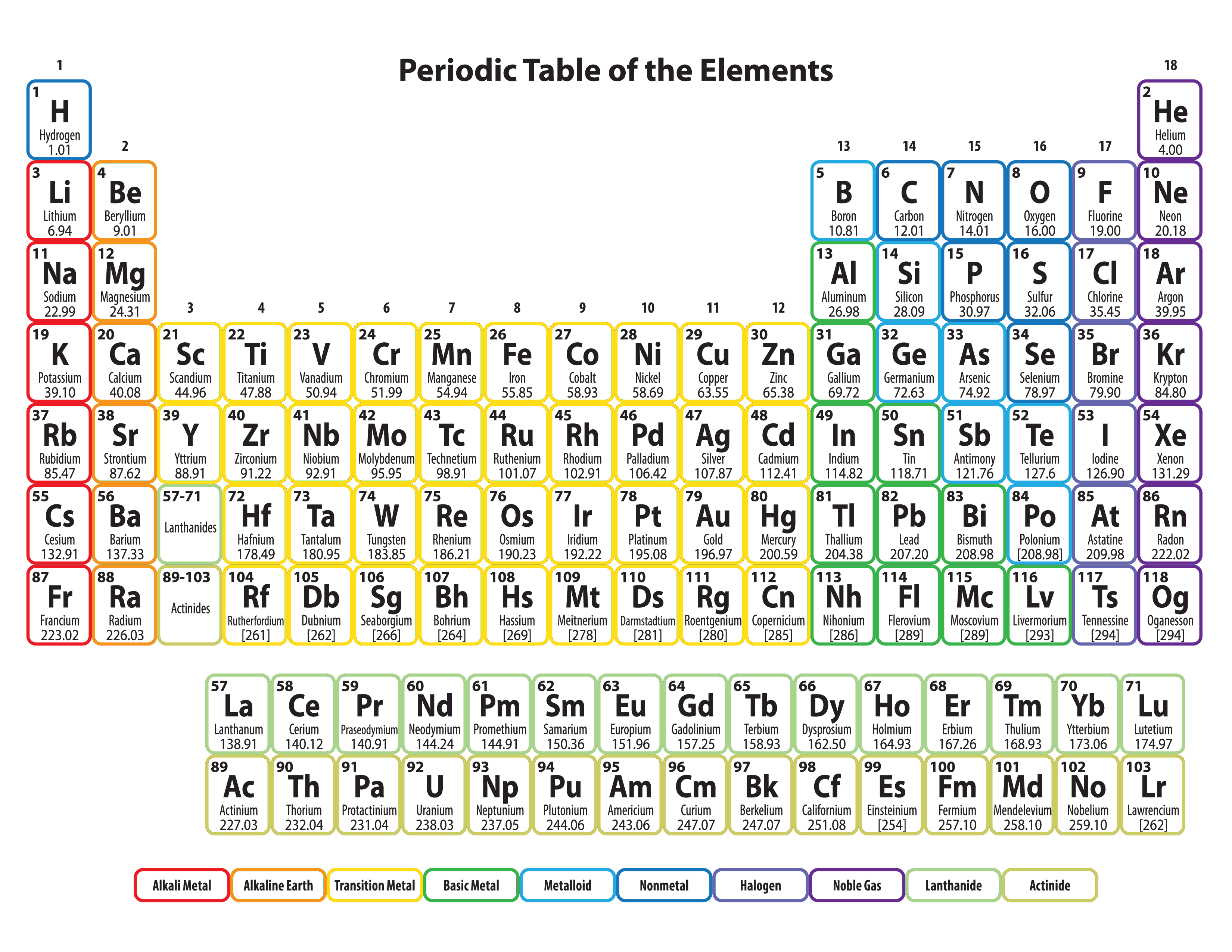
Cerium signifies the entry of electrons into the 4f orbital, resulting in the lanthanide series of 4f-inner transition elements. With n = 6, the sixth period has 32 elements, with electrons filling the 6s, 4f, 5d, and 6p orbitals.The 5p orbital is completely filled by Xenon at the end of the period. The 4d transition series, which begins with the Yttrium, dominates this time. The level 5s are filled first in the fifth period with n = 5.Scandium is the first of the three-dimensional transition elements. However, we know that the 3d orbital must be full before the 4p orbital can be filled. The level 4s are filled first in the fourth period with n = 4.There are eight elements in this period as well. The third period begins with Sodium and finishes with Argon, filling the 3s and 3p orbitals in that order.The second period begins with Lithium and Beryllium, both of which have three and four electrons, respectively, and so the final electrons reach the level twos.So the first energy level (K shell) can hold up to 2 electrons, the second (L shell) up to 8 electrons, the third (M shell) up to 18 electrons, and so on. 2n 2, where n is the energy level, is the greatest number of electrons that a given energy level can allow.The number of electrons that can be accommodated by different energy levels varies.The period of the element is the value of n, the primary quantum number, for the valence shell.Predicting the qualities of a group of elements (elements with similar electron configurations tend to exhibit similar properties).As a result, electron configurations can be used for: As a result, sodium’s abbreviated electron configuration is 3s 1 (the electron configuration of neon is 1s 2 2s 2 2p 6, which can be abbreviated to 2s 2 2p 6). In shortened notation, the sequence of entirely filled subshells that correspond to a noble gas’s electronic configuration is replaced by the noble gas’s symbol in square brackets. In such instances, a shortened or condensed notation may be employed instead of the normal notation. Long electron configurations are typically produced by the conventional notation (especially for elements having a relatively large atomic number). Average and Instantaneous Rate of ChangeĪn electronic configuration, also known as an electronic structure, is the arrangement of electrons at different energy levels around an atomic nucleus.Class 11 NCERT Solutions - Chapter 3 Trigonometric Function - Exercise 3.1.Augmented Assignment Operators in Python.Importance of Chemistry in Everyday Life.Class 11 NCERT Solutions - Chapter 7 Permutations And Combinations - Exercise 7.1.Difference Between Mean, Median, and Mode with Examples.What is the Difference between Interactive and Script Mode in Python Programming?.
ISRO CS Syllabus for Scientist/Engineer Exam.ISRO CS Original Papers and Official Keys.GATE CS Original Papers and Official Keys.


 0 kommentar(er)
0 kommentar(er)
